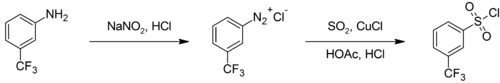Sulfur dioxide
| Sulfur dioxide | |
|---|---|
 |
 |
|
Other names
Sulfur(IV) oxide
Sulfurous anhydride |
|
| Identifiers | |
| CAS number | 7446-09-5 |
| PubChem | 1119 |
| ChemSpider | 1087 |
| EC number | 231-195-2 |
| RTECS number | WS4550000 |
|
SMILES
O=S=O
|
|
| Properties | |
| Molecular formula | SO2 |
| Molar mass | 64.07 g/mol |
| Appearance | colorless gas |
| Density | 2.551 g/L (gas) 1.46 g/cm3 (liquid, −10 °C) |
| Melting point |
−75.5 °C, 198 K, -104 °F |
| Boiling point |
−10.0 °C, 263 K, 14 °F |
| Solubility in water | 22.97 g/100 mL (0 °C) 11.58 g/100 mL (20 °C) 9.4 g/100 mL (25 °C) [1] |
| Solubility | very soluble in acetone, methyl isobutyl ketone, acetic acid, alcohol soluble in sulfuric acid |
| Acidity (pKa) | 1.81 |
| Viscosity | 0.403 cP (0 °C) |
| Structure | |
| Molecular shape | Bent, C2v |
| Dipole moment | 1.62 D |
| Hazards | |
| MSDS | ICSC 0074 |
| EU Index | 016-011-00-9 |
| EU classification | Toxic (T) Corrosive (C) |
| R-phrases | R23 R34 |
| S-phrases | (S1/2) S9 S26 S36/37/39 S45 |
| NFPA 704 |
 0
3
0
|
| Flash point | Non-flammable |
| LD50 | 3000 ppm (30 min inhaled, mouse) |
| Related compounds | |
| Other cations | Selenium dioxide Tellurium dioxide |
| Related sulfur oxides | Sulfur monoxide Sulfur trioxide |
| Related compounds | sulfurous acid ozone |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Sulfur dioxide (also sulphur dioxide) is the chemical compound with the formula SO2. It is produced by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel. Further oxidation of SO2, usually in the presence of a catalyst such as NO2, forms H2SO4, and thus acid rain.[2] Sulfur dioxide emissions are also a precursor to particulates in the atmosphere. Both of these impacts are cause for concern over the environmental impact of these fuels.
Contents |
Structure and bonding
SO2 is a bent molecule with C2v symmetry point group. In terms of electron-counting formalisms, the sulfur atom has an oxidation state of +4, a formal charge of 0. It is surrounded by 5 electron pairs and can be described as a hypervalent molecule. From the perspective of molecular orbital theory, most of these valence electrons are engaged in S–O bonding.

Although sulfur and oxygen both have six valence electrons, the molecular bonds in SO2 are not the same as those in ozone. The S–O bonds are shorter in SO2 (143.1 pm) than in sulfur monoxide, SO (148.1 pm), whereas the O–O bonds are longer in ozone (127.8 pm) than in dioxygen, O2 (120.7 pm). The mean bond energy is greater in SO2 (548 kJ/mol) than in SO (524 kJ/mol), whereas it is less in O3 (297 kJ/mol) than in O2 (490 kJ/mol). These pieces of evidence lead chemists to conclude that the S–O bonds in sulfur dioxide have a bond order of at least 2, unlike the O–O bonds in ozone, which have a bond order of 1.5.[3]
Reactions
Treatment of basic solutions with sulfur dioxide affords sulfite salts:
- SO2 + 2 NaOH → Na2SO3 + H2O
Featuring sulfur in the +4 oxidation state, sulfur dioxide is a reducing agent. It is oxidized by halogens to give the sulfuryl halides, such as sulfuryl chloride:
- SO2 + Cl2 → SO2Cl2
However, on rare occasions, it can also act as an oxidising agent: in the Claus process, sulfur dioxide is reduced by hydrogen sulfide to give elemental sulfur:
- SO2 + 2 H2S → 3 S + 2 H2O
Sulfur dioxide can react with 1,3-dienes in a cheletropic reaction.
Sulfur dioxide can bind to metal ions as a ligand, typically where the transition metal is in oxidation state 0 or +1.[4] Many different bonding modes (geometries) are recognized, but in most cases the ligand is monodentate, attached to the metal through sulfur, which can be either planar and pyramidal η1.[4]
Preparation
Sulfur dioxide can be prepared by burning sulfur:
- S8 + 8 O2 → 8 SO2
The combustion of hydrogen sulfide and organosulfur compounds proceeds similarly.
- 2 H2S + 3 O2 → 2 H2O + 2 SO2
The roasting of sulfide ores such as pyrite, sphalerite, and cinnabar (mercury sulfide) also releases SO2:
- 4 FeS2 + 11 O2 → 2 Fe2O3 + 8 SO2
- 2 ZnS + 3 O2 → 2 ZnO + 2 SO2
- HgS + O2 → Hg + SO2
Sulfur dioxide is a by-product in the manufacture of calcium silicate cement: CaSO4 is heated with coke and sand in this process:
- 2 CaSO4 + 2 SiO2 + C → 2 CaSiO3 + 2 SO2 + CO2
Action of hot sulfuric acid on copper turnings produces sulfur dioxide.
- Cu (s) + 2 H2SO4 (aq) → CuSO4 (aq) + SO2 (g) + 2 H2O (l)
It can also be prepared with sodium metabisulfite:
- H2SO4 (aq) + Na2S2O5 (aq) → 2 SO2 (g) + Na2SO4 (s) + H2O (l)
This is an exothermic reaction.
Uses
Precursor to sulfuric acid
Sulfur dioxide is an intermediate in the production of sulfuric acid, being converted to sulfur trioxide, and then to oleum, which is made into sulfuric acid. Sulfur dioxide for this purpose is made when sulfur combines with oxygen. The method of converting sulfur dioxide to sulfuric acid is called the contact process. Several billion kilograms are produced annually for this purpose.
As a preservative
Sulfur dioxide is sometimes used as a preservative for dried apricots and other dried fruits owing to its antimicrobial properties, and it is sometimes called E220 when used in this way. As a preservative, it maintains the appearance of the fruit and prevents rotting.
In winemaking
Sulfur dioxide is an important compound in winemaking, and is designated as parts per million in wine, E number: E220.[5] It is present even in so-called unsulphurated wine at concentrations of up to 10 milligrams per litre.[6] It serves as an antibiotic and antioxidant, protecting wine from spoilage by bacteria and oxidation. It also helps to keep volatile acidity at desirable levels. Sulfur dioxide is responsible for the words "contains sulfites" found on wine labels. Wines with SO2 concentrations below 10 ppm do not require "contains sulfites" on the label by US and EU laws. The upper limit of SO2 allowed in wine in the US is 350 ppm; in the EU it is 160 ppm for red wines and 210 ppm for white and rosé wines. In low concentrations SO2 is mostly undetectable in wine, but at over 50ppm, SO2 becomes evident in the nose and taste of wine.
SO2 is also a very important element in winery sanitation. Wineries and equipment must be kept clean, and because bleach cannot be used in a winery, a mixture of SO2, water, and citric acid is commonly used to clean and sanitize equipment. Compounds of ozone (O3) are now used extensively as cleaning products in wineries due to their efficiency, and because these compounds do not affect the wine or equipment.
As a reducing agent
Sulfur dioxide is also a good reductant. In the presence of water, sulfur dioxide is able to decolorize substances. Specifically it is a useful reducing bleach for papers and delicate materials such as clothes. This bleaching effect normally does not last very long. Oxygen in the atmosphere reoxidizes the reduced dyes, restoring the color. In municipal wastewater treatment sulfur dioxide is used to treat chlorinated wastewater prior to release. Sulfur dioxide reduces free and combined chlorine to chloride.[7]
Biochemical and biomedical roles
Sulfur dioxide is toxic in large amounts. It or its conjugate base bisulfite is produced biologically as an intermediate in both sulfate-reducing organisms and in sulfur oxidizing bacteria as well. Sulfur dioxide has no role in mammalian biology. Sulfur dioxide blocks nerve signals from the pulmonary stretch receptors (PSR's) and abolishes the Hering-Breuer inflation reflex.
As a refrigerant
Being easily condensed and possessing a high heat of evaporation, sulfur dioxide is a candidate material for refrigerants. Prior to the development of freons, sulfur dioxide was used as a refrigerant in home refrigerators.
As a reagent and solvent in the laboratory
Sulfur dioxide is a versatile inert solvent that has been widely used for dissolving highly oxidizing salts. It is also used occasionally as a source of the sulfonyl group in organic synthesis. Treatment of aryl diazonium salts with sulfur dioxide and cuprous chloride affords the corresponding aryl sulfonyl chloride, for example:[8]
Emissions

According to the United States Environmental Protection Agency (EPA) (as presented by the 2002 World Almanac or in chart form[9]), the following amount of sulfur dioxide was released in the U.S. per year, measured in thousands of short tons:
- 1999 18,867
- 1998 19,491
- 1997 19,363
- 1996 18,859
- 1990 23,678
- 1980 25,905
- 1970 31,161
Sulfur dioxide emissions are a precursor to acid rain and atmospheric particulates. Due largely to the US EPA’s Acid Rain Program, the U.S. has witnessed a 33 percent decrease in emissions between 1983 and 2002. This improvement resulted in part from flue gas desulfurization, a technology that enables SO2 to be chemically bound in power plants burning sulfur-containing coal or oil. In particular, calcium oxide (lime) reacts with sulfur dioxide to form calcium sulfite:
- CaO + SO2 → CaSO3
Aerobic oxidation of the CaSO3 gives CaSO4, anhydrite. Most gypsum sold in Europe comes from flue gas desulfurization.
Sulfur can be removed from coal during the burning process by using limestone as a bed material in Fluidized bed combustion[10]
Sulfur can also be removed from fuels prior to burning the fuel. This prevents the formation of SO2 because there is no sulfur in the fuel from which SO2 can be formed. The Claus process is used in refineries to produce sulfur as a byproduct. The Stretford process has also been used to remove sulfur from fuel. Re-Dox processes using iron oxides can also be used, for example, Lo-Cat [11] or Sulferox [12].
Fuel additives, such as calcium additives and magnesium oxide, are being used in gasoline and diesel engines in order to lower the emission of sulfur dioxide gases into the atmosphere.[13]
As of 2006, China is the world's largest sulfur dioxide polluter, with 2005 emissions estimated to be 25.49 million tons. This amount represents a 27% increase since 2000, and is roughly comparable with U.S. emissions in 1980.[14]
Safety
Inhaling sulfur dioxide is associated with increased respiratory symptoms and disease, difficulty in breathing, and premature death.[15] In 2008, the American Conference of Governmental Industrial Hygienists reduced the Short-term exposure limit from 5ppm to 0.25ppm. The OSHA PEL is currently set at 5ppm (13 mg/m3) time weighted average. NIOSH has set the IDLH at 100ppm.[16]
- Food additive
In the United States, the Center for Science in the Public Interest lists the two food preservatives, sulfur dioxide and sodium bisulfate, as being safe for human consumption except for certain individuals who may be sensitive to it, especially in large amounts.[17]
References
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0070494398
- ↑ Holleman, A. F.; Wiberg, E. (2001), Inorganic Chemistry, San Diego: Academic Press, ISBN 0-12-352651-5
- ↑ Greenwood, Norman N.; Earnshaw, Alan. (1997), Chemistry of the Elements (2nd ed.), Oxford: Butterworth-Heinemann, p. 700, ISBN 0080379419
- ↑ 4.0 4.1 Greenwood, Norman N.; Earnshaw, A. (1984), Chemistry of the Elements, Oxford: Pergamon, pp. 824–32, ISBN 0-08-022057-6
- ↑ Current EU approved additives and their E Numbers, The Food Standards Agency website.
- ↑ Sulphites in wine, MoreThanOrganic.com.
- ↑ Tchobanoglous, George. Wastewater Engineering. 3rd ed. New York: Mc Graw Hill, 1979.
- ↑ R. V. Hoffman (1990), "m-Trifluoromethylbenzenesulfonyl Chloride", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV7P0508; Coll. Vol. 7: 508
- ↑ National Trends in Sulfur Dioxide Levels, United States Environmental Protection Agency.
- ↑ [Lindeburg, Michael R. Mechanical Engineering Reference Manual for the PE Exam. Belmont, C.A.: Professional Publications, Inc, 2006. p. 27-3. ISBN 978-1-59126-049-3..]
- ↑ Lo-Cat Process
- ↑ Sulferox
- ↑ http://www.sfainternational.com/library/Marine%20Emissions%20Abatement.pdf, Page 6.
- ↑ China has its worst spell of acid rain, United Press International.
- ↑ [1]U.S. Environmental Protection Agency
- ↑ NIOSH Pocket Guide to Chemical Hazards
- ↑ "Center for Science in the Public Interest - Chemical Cuisine". http://www.cspinet.org/reports/chemcuisine.htm. Retrieved March 17, 2010.
See also
- Sulfur trioxide
- Sulfur-iodine cycle
- National Ambient Air Quality Standards
- Homer City Generating Station
External links
- United States Environmental Protection Agency Sulfur Dioxide page
- International Chemical Safety Card 0074
- IARC Monographs. "Sulfur Dioxide and some Sulfites, Bisulfites and Metabisulfites" v54. 1992. p131.
- NIOSH Pocket Guide to Chemical Hazards
- Food Intolerance Network - Sulfite factsheet
- Sulfur Dioxide, Molecule of the Month